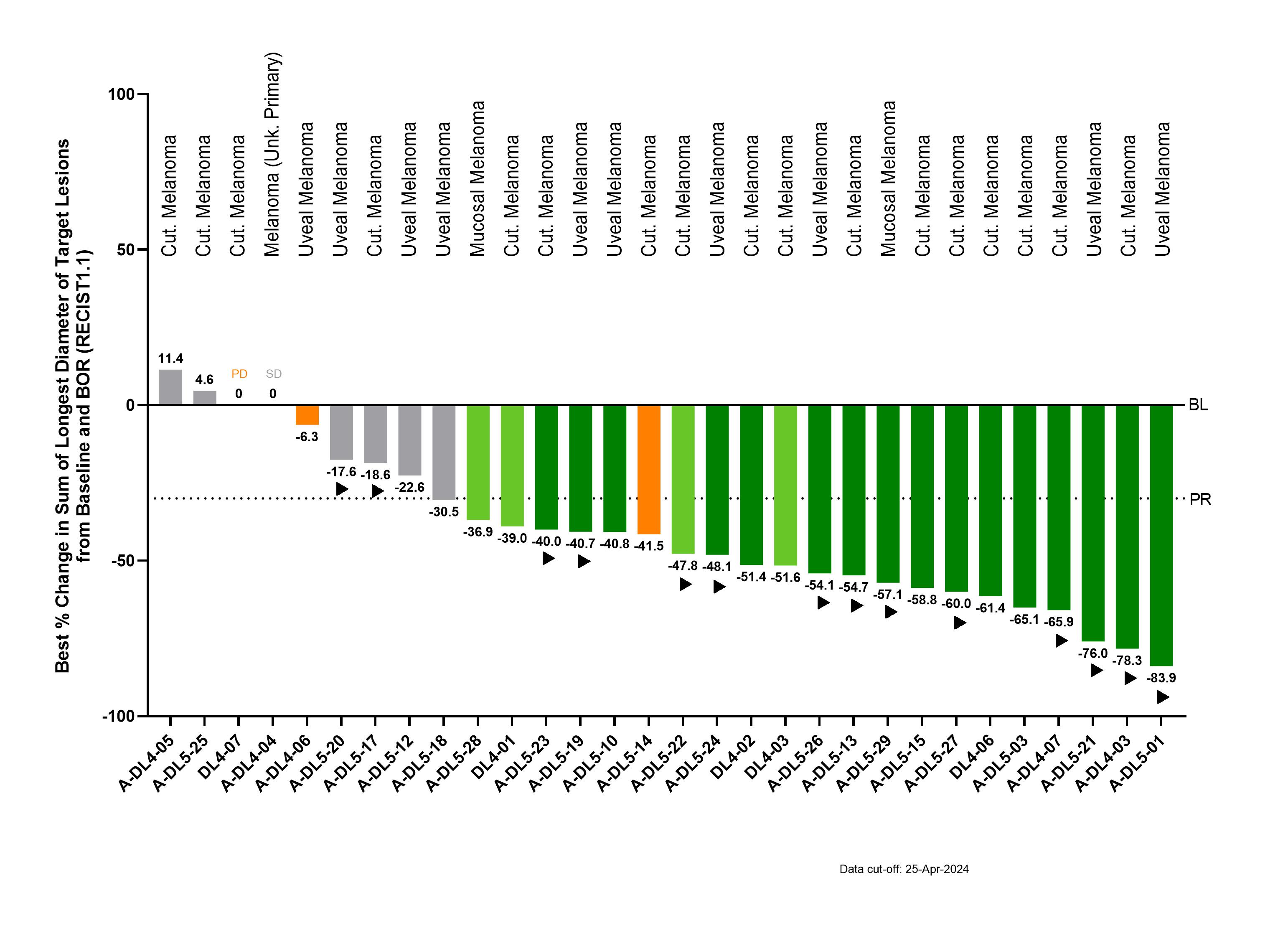
The company provided an updated clinical data on ACTengine® IMA203 targeting PRAME from the ongoing Phase 1 trial at the recommended Phase 2 dose (RP2D, 1 to 10 billion total TCR-T cells) in 30 heavily pretreated metastatic melanoma patients evaluable for efficacy. The treated patient population consisted of patients with a median of 3 lines of prior systemic treatments, including 17 cutaneous melanoma patients, 10 uveal melanoma patients, 2 mucosal melanoma patients and 1 patient with melanoma of unknown primary. As of the April 25, 2024 data cut-off, treatment with IMA203 monotherapy in the efficacy population demonstrated a confirmed objective response rate of 55% (16/29), a disease control rate of 90% (27/30), and tumor shrinkage in 87% (26/30) of patients. The median duration of response was 13.5 months (minimum 1.2+, maximum 21.5+ months) including 11 of 16 confirmed objective responses ongoing at data cut-off and the longest duration of response ongoing at >21 months after infusion. Confirmed response rates were similar across all melanoma subtypes (56% (9/16) in cutaneous melanoma; 54% (7/13) in other melanoma subtypes). To date, IMA203 has maintained a favorable safety profile with no treatment-related grade 5 events in the safety population (N=65 patients across all dose levels and all tumor types).
More information and details on the IMA203 clinical data update in melanoma are available in the Immatics corporate presentation. The next data update with translational and clinical data for IMA203 is planned for the second half of 2024 at a medical conference. Immatics’ late-stage clinical cell therapy development is supported by its differentiated manufacturing related to timeline, capabilities and facilities. ACTengine® IMA203 cell therapy products are manufactured within 7 days, followed by a 7-day QC release testing at a success rate of >95% to reach the target dose. The company has also recently completed construction of a ~100,000 square foot R&D and GMP manufacturing facility with a modular design for efficient and cost-effective scalability intended to serve early-stage and Phase 2/3 clinical trials, as well as initial commercial supply. The new site will start GMP manufacturing of cell therapy products in early 2025. Meanwhile, the existing GMP facility, which is run in collaboration with UT Health, will remain active until YE 2025 and will also initially serve the Phase 2/3 registrational trial.
Following a productive interaction with the FDA, Immatics plans to initiate a randomized Phase 2/3 trial in 4Q 2024 for IMA203 in patients with second-line or later (2L+) cutaneous melanoma, potentially also including uveal melanoma patients. The Phase 2/3 trial is expected to assess IMA203 targeting PRAME in HLA-A*02:01-positive cutaneous melanoma patients versus a control arm. This approach is consistent with the FDA’s “one-trial” approach2, i.e., a single randomized controlled trial to support accelerated approval and the verification of clinical benefit to achieve full approval. The high prevalence of PRAME (≥95%) in cutaneous melanoma may enable the company to enroll patients without PRAME pre-testing. This would enhance trial operations and would remove the need to develop a companion diagnostic for PRAME testing in this indication. The full trial design is currently being developed and is subject to further alignment with the FDA as part of the ongoing discussions. Further details of the final clinical trial design will be provided in the second half of 2024. IMA203 is being developed to treat patients with metastatic melanoma, a prevalent cancer type with increasing incidence both inside and outside the United States. Currently, eligible PRAME-positive melanoma patients for the ongoing trials, i.e., 2L+, HLA-A*02:01 positive, include ~3,000 cutaneous melanoma patients and ~300 eligible uveal melanoma patients3 in the US.
As of the previously reported interim clinical update from 2023, the first data on the company’s second-generation product candidate, IMA203CD8 (consisting of PRAME-specific functional CD8+ and CD4+ cells), demonstrated 56% (5/9) cORR with enhanced pharmacology compared to IMA203. mDOR was not reached (min 2.0+ months, max 11.5+ months) at a mFU of 4.8 months. As of the reported September 30, 2023, cut-off date, IMA203CD8 (GEN2) exhibited a manageable tolerability profile. For IMA203CD8 (GEN2), Immatics cleared dose level 4a (DL4a, up to ~1.6×109 TCR-T cells) in December 2023. Immatics plans to continue dose escalation with the goal to define the optimal dose for further development. In addition to treating melanoma patients, Immatics has also started to expand its clinical footprint outside of melanoma to address a broader patient population with a particular focus on ovarian and uterine cancers. A next data update for IMA203CD8 (GEN2) is planned for the second half of 2024.
Immatics’ T cell engaging receptor (TCER®) candidates are next-generation, half-life extended TCR Bispecific molecules. They are
